| CAS
NO. |
80210-62-4
(Base)
87239-81-4 (proxetil) |
|
| EINECS NO. |
|
| FORMULA |
C21H27N5O9S2 |
| MOL
WT. |
557.59 |
|
H.S.
CODE
|
|
|
TOXICITY
|
|
| SYNONYMS |
Vantin; Orelox;
|
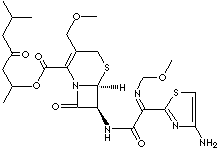
| (RS)-1(isopropoxycarbonyloxy)ethyI (+)-(6R,7R)-7-[2-(
2-amino-4-thiazolyl)-2-[(Z) methoxyimino] acetamido]-3-
methoxymethyl-8-oxo-5-thia-1-azabicyclo [4.2.0]oct-2-ene-2- carboxylate; |
|
CLASSIFICATION
|
ANTIBOITICS
/ CEPHALOSPORINS
/
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
White to yellowish powder |
| MELTING POINT |
|
| BOILING
POINT |
|
| SPECIFIC GRAVITY |
|
| SOLUBILITY
IN WATER |
|
| pH |
|
| VAPOR DENSITY |
|
|
AUTOIGNITION
|
|
|
NFPA
RATINGS
|
|
|
REFRACTIVE
INDEX
|
|
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary conditions. |
|
GENERAL
DESCRIPTION & APPLICATIONS
|
|
Cephalosporin: any of a group of broad-spectrum derived from species of fungi of
the genus Cephalosporium and are related to the penicillins in both structure
and mode of action but relatively penicillinase-resistant antibiotics. These
antibiotics have low toxicity for the host, considering their broad
antibacterial spectrum. They have the active nucleus of beta-lactam ring which
results in a variety of antibacterial and pharmacologic characteristics when
modified mainly by substitution at 3 and 7 positions. Their antibacterial activities
result from the inhibition of mucopeptide synthesis in the cell wall. They are
widely used to treat gonorrhea, meningitis, pneumococcal, staphylococcal and
streptococcal infections. The cephalosporin class of antibiotics is usually
divided into generations by their antimicrobial properties. Three generations of
cephalosporins are recognized and the fourth has been grouped. Each newer
generation of cephalosporins has broader range of activity against gram-negative
organisms but a narrower range of activity against gram-positive organisms than
the preceding generation. The newer agents have much longer half-lives resulting
in the decrease of dosing frequency. Accordingly, the third-generation
cephalosporins can penetrate into tissues well, and thus antibiotic levels are
good in various body fluids. Third-generation cephalosporins are more active
against gram-negative organisms but less active against gram-positive organisms
than second-generation agents; examples are cefoperazone, cefotaxime,
ceftriaxone, ceftazidime, ceftizoxime, and moxalactam. Cefpodoxime proxetil is a broad-spectrum beta-lactamase–resistant cephalosporin;
administered orally. The chemical name is (RS)-1(isopropoxycarbonyloxy) ethyI
(+)- (6R,7R)-7-[2-( 2-amino-4-thiazolyl)- 2-[(Z)-methoxy imino] acetamido]-3-
methoxymethyl-8-oxo-5-thia-1-azabicyclo [4.2.0]oct-2-ene-2- carboxylate. |
| SALES
SPECIFICATION |
|
APPEARANCE
|
White to yellowish powder |
|
ASSAY |
690
- 800µg/mg
min (cefpodoxime),
90.0~102.0% (cefpodoxime proxetil) |
|
MOISTURE |
3.0%
max |
|
RELATED
SUBSTANCE
|
6.0%
max
|
|
pH
|
6
- 8 (1% aq sol.) |
|
HEAVY
METALS
|
20ppm
max
|
|
OPTICAL
ROTATION
|
+35°
~ +45° |
| TRANSPORTATION |
| PACKING |
5kgs
- 20kgs |
| HAZARD CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
|
Price:

|
